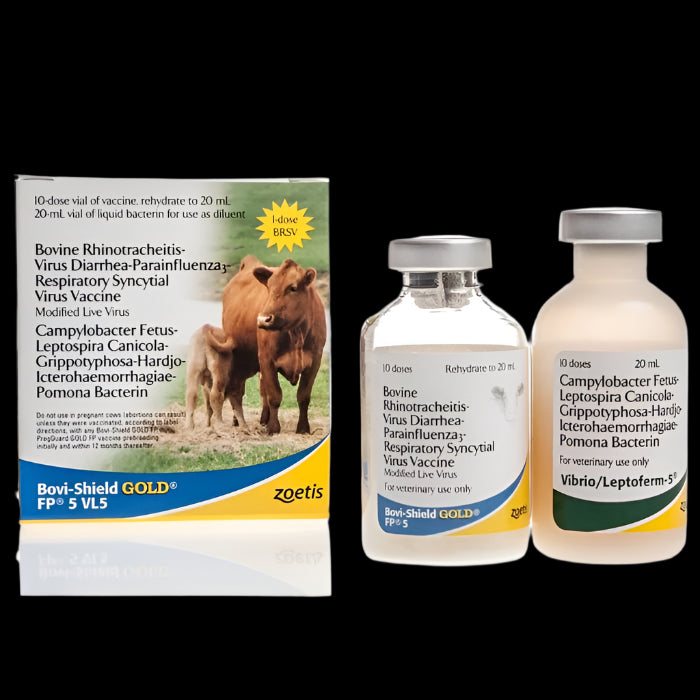
BOVI-SHIELD GOLD FP 5 VL5 is a modified-live vaccine that provides protection from bovine viral diarrhea (BVD) Types 1 and 2 viruses, infectious bovine rhinotracheitis (IBR) virus, campylobacteriosis and five serovars of leptospirosis. In addition, Bovi-Shield GOLD FP 5 VL5 protects against Campylobacteriosis (Vibriosis) caused by Campylobacter fetus. Bovi-Shield GOLD FP 5 VL5 is a combination vaccine covered under Zoetis' Fetal Protection (FP) Guarantee, which assures protection against IBR abortion and BVD (Types 1 and 2) persistently infected calves. A 12 month duration of immunity has been demonstrated against IBR-induced abortion and persistently infected calves caused by BVD Types 1 and 2.
This vaccine may be administered to pregnant cows, or calves nursing pregnant cows, provided cows were vaccinated, according to label directions, with any Bovi-Shield GOLD FP vaccine within the past 12 months. Failure to follow these directions can result in abortion. Protects against fetal infection caused by (BVD) Types 1 and 2 viruses. Bovi-Shield GOLD FP 5 VL5 contains a modified live virus (MLV) and requires reconstitution with the included liquid bacterin (Vibrio/Leptoferm-5) prior to administration. It should be administered intramuscularly in the neck region. Do not administer within 21 days before slaughter. Do not vaccinate calves under 3 months of age. Annual revaccination is recommended.
The freeze-dried vaccine is a preparation of modified live virus (MLV) strains of IBR, BVD (Types 1 and 2), PI3 and BRSV. The Campylobacter bacterin is an inactivated suspension of C. fetus. It is combined with inactivated Leptospira bacterin prepared from whole cultures of the agents indicated. The Campylobacter-Leptospira bacterin is supplied as a diluent for the IBR-BVD-PI3-BRSV vaccine.
Bovi-Shield GOLD FP 5 VL5 is for the vaccination of healthy beef and dairy cows and heifers prior to breeding as an aid in the prevention of the following diseases of cattle:
Respiratory diseases:
1. Infectious bovine rhinotracheitis (IBR)
2. Bovine viral diarrhea (BVD Types 1 and 2)
3. Bovine viral diarrhea (BVD Type 2 testicular infection)
4. Parainfluenza3 (PI3)
5. Bovine respiratory syncytial virus (BRSV)
Leptospirosis caused by:
1. Leptospira canicola
2. L. grippotyphosa
3. L. hardjo
4. L. icterohaemorrhagiae
5. L. pomona
Dosage
2 mL dose to all breeding cows and heifers one month prior to breeding or being added to the herd, followed by single doses of Vibrio/Leptoferm 5 and Bovi-shield BRSV 3-4 weeks later. Annual revaccination is recommended. May be administered to pregnant cattle and calves nursing pregnant cows as they were vaccinated according to label directions. Not for calves under 3 months of age. 21 day slaughter withhold.
Indications
Prevents persistently infected calves caused by BVD Types 1 and 2 viruses; aids in preventing abortion caused by IBR virus; respiratory disease caused by IBR virus, BVD Types 1 and 2 viruses, parainfluenza 3 (PI3) virus and bovine respiratory syncytial virus (BRSV); BVD Type 2 testicular infection; campylobacteriosis (vibriosis) caused by Campylobacter fetus (vibrio) and leptospirosis caused by the five Leptospira serovars indicated above.
IMPORTANT SAFETY INFORMATION
Do not use in pregnant cattle (abortions can result) unless they were vaccinated, according to label directions, with any BOVI-SHIELD GOLD FP or PREGGUARD GOLD FP vaccine prebreeding initially and within 12 months thereafter. Do not use in calves nursing pregnant cows unless their dams were vaccinated within the past 12 months as described above. To help ensure safety in pregnant cattle, heifers must receive at least 2 doses of any BOVI-SHIELD GOLD FP or PREGGUARD GOLD FP vaccine with the second dose administered at least 30 days prebreeding.
Bovi-Shield Gold FP 5+VL5 Highlights:
- Manufacturer: Zoetis
- Bovine combination vaccine for respiratory and reproductive disease protection
- Protects against IBR, BVD Types 1 and 2, PI3, BRSV, Vibrio and 5 strains of Leptospira
- Modified live virus vaccine, requires mixing prior to use
- For use in healthy beef and dairy cattle at least 3 months of age
- 21 day slaughter withdrawal period
- May also be administered to calves nursing pregnant cows provided their dams were vaccinated within the past 12 months as described above